

SO2 Lewis Structure- Key Points The electron geometry of SO2 is formed in the shape of a trigonal planner. Looking at the positions of other atomic nuclei around the central determine the molecular geometry.Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons.According to VSEPR Theory, the molecular geometry will be trigonal pyramidal, AX3E1. The arsenic atom is surrounded by 4 regions of electron density, which means that its has a steric number equal to 4. The resulting molecular shape is bent with an H-O-H angle of 104.5°. These are arranged in a tetrahedral shape. Water has 4 regions of electron density around the central oxygen atom (2 bonds and 2 lone pairs). Therefore geometry should be tetrahedral. Summation of number of sigma bonds and lone-pairs around phosphorus atom is four. Therefore, shape of PH3 is trigonal pyramidal.

There are three sigma bonds and one lone-pair around phosphorus atom. Note that in the given Lewis structure, 3 of the 4 atoms bears a formal charge. Molecule or IonAtomsTotal Valence Electrons3 HCF4C 324 FNO3-N24 What is the electronic geometry of no − 3?īecause there are 3 regions of electron density around the central nitrogen, trigonal planar geometry is suggested. The ammonia molecule has a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom. The I-F bond angle is 82 degrees in the square pyramidal IF5 molecular geometry. Because the center atom, Iodine, has five I-F bonds with the five fluorine atoms surrounding it. What is the molecular geometry of if 5?Īccording to the VSEPR theory, the IF5 molecule ion possesses square pyramidal molecular geometry. How is CF4 nonpolar?ĬF4 is in a tetrahedral molecular shape, so all the dipoles of the 4 polar C-F bonds cancel each other out, resulting in an overall nonpolar molecule. It consists of four hydrogen atoms and one carbon atom and is the simplest alkane. Methane is a simplest of the saturated hydrocarbons with a chemical formula CH4. … That is why methane assumes tetrahedral geometry. The four C-H bonds in methane are held at an angle of 109∘-28′ because this is the only angle in space at which repuslions between the four shared pairs of electrons is minimum. 2 : relating to, forming, or having the form of a tetrahedron.
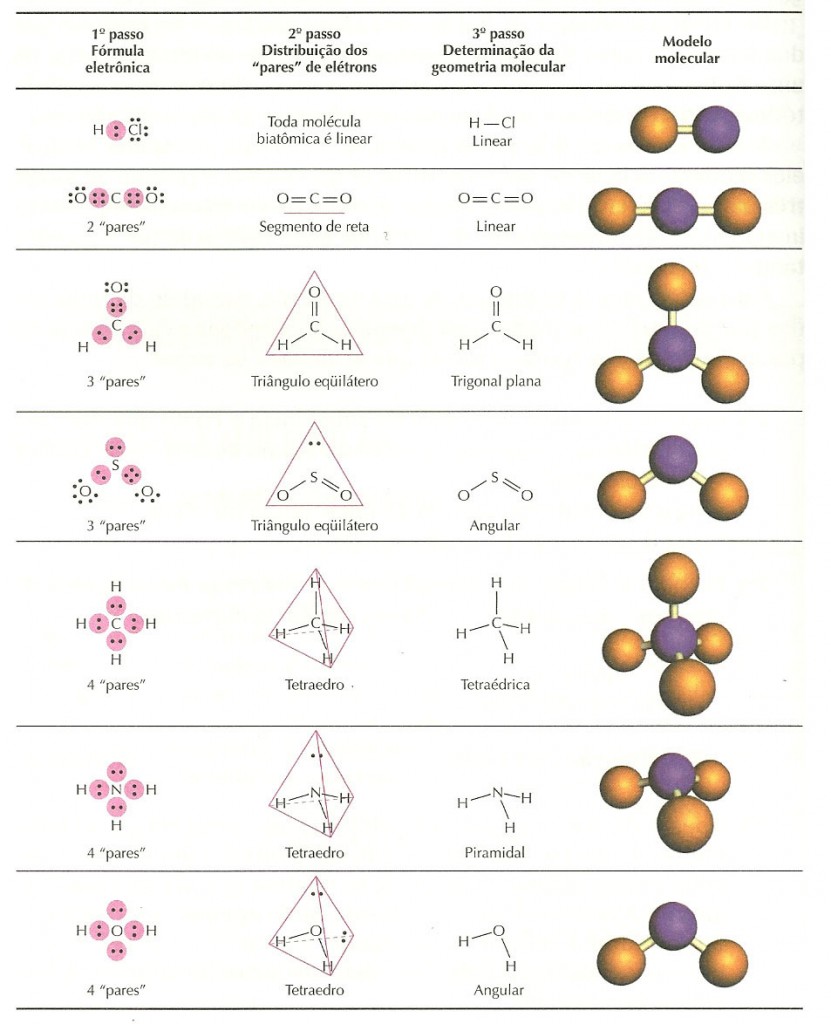
Which of the following is not tetrahedral?ĭefinition of tetrahedral 1 : being a polyhedral angle with four faces. Tetrahedral molecules are nonpolar if the four surrounding atoms are the same, in the cases of methane and ammonium, and have a bond angle of 109.5˚. Methane has a central carbon atom ringed by four hydrogens, which form the “spokes” of the tetrahedral molecule. What is the example of tetrahedral?Įxamples include methane ( CH4 ) and ammonium ( NH+4 ). The bond angles are almost approximately 109.50 degrees. So, according to the chart, we can see that SO42- has a tetrahedral structure. … The bromine atom will be bonded to each of the four fluorine atoms via single bonds for a total of 8 of the 36 valence electrons. Start from the Lewis structure of the tetrafluoroborate ion, BrF−4. Is brf4+ a tetrahedral?Įxplanation: The answer is A) square planar. Thus, the geometry will be tetrahedral as 4 bond pairs and 0 lone pairs will be there. Thus, all four electrons form a single bond with each fluorine atom each and no lone pair will be there on central atom C. What is the 3 D shape of CF4?įluorine has a valency of 1 as it is only one electron short of noble gas configuration. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. If these are all bond pairs the molecular geometry is tetrahedral (e.g. Is CH4 trigonal?įor example four electron pairs are distributed in a tetrahedral shape. In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. Hence the shape of \ will be tetrahedral. … Since all the valence electrons on Boron participate in bonding, there will be no lone-pair electrons on Boron.
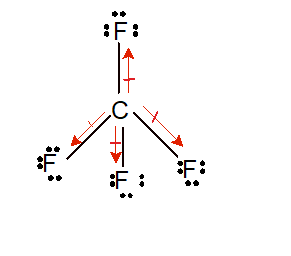
Each fluorine atom contains an unpaired electron on one of the p-orbitals. We have four fluorine atoms in the molecule. It turns out that methane is tetrahedral, with 4 equal bond angles of 109.5° and 4 equal bond lengths, and no dipole moment. 34 Which of the following is a tetrahedral species? Is CH4 tetrahedral?


 0 kommentar(er)
0 kommentar(er)
